It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. Q2. It is a reaction that involves alkyl and aryl halides. B.WurtzFittig Organic reactions have a restricted number of applications. WebThe Wurtz reaction is an organic chemical process that is applied in laboratories to create alkanes. Here, a large alkane molecule is developed by joining two compounds of alkyl halide and eradicating the halogen atoms in the form of sodium halide. It is a method to synthesize higher alkanes by a reaction between alkyl halides and metallic sodium in the presence of dry ether. Give a name of a reaction other than the Wurtz reaction to increasing the length of Carbon atoms? As a result of the Wurtz reaction process, the necessary alkane product is generated. In this mechanism, two free phenyl radicals react to form benzene and a free phenylene anion. We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. In the presence of dry ether, this combination gives higher alkanes as a product. Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. It involves the reaction between an alkyl halide and an aryl halide in the presence of sodium metal and dry ether to yield a substituted aromatic compound. What is the IUPAC name of the lowest molecular weight alkane that contains a quaternary carbon? Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Because sodium reacts violently with oxygen and moisture, an anhydrous state is required.  Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. What is the chemical reaction's name? WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Wurtz Reaction Click Start Quiz to begin! The sodium metal used in the reaction is a highly reactive element and thus A minimum of two carbon atoms must be present in the process, which does not apply to methane. The Vapour phase is considered a suitable phase for free radicals. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. Na+ and X combine to form a salt. Alkyl free radicals are formed as a result of this. Explanation: The KMnO4 solution gives the test for unsaturation of organic compounds. The Wurtz-Fittig reaction is a chemical process that produces substituted aromatic compounds from aryl Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. Why WurtzFittig reaction is not suitable for tertiary alkyl halide? In this lecture we are providing complete information about Wurtz Fittig Reaction. Answer: The structure of C3H4 (allene) molecule is CH2=C=CH2. Applications of WurtzFittig reactions are limited.
Wurtz reaction is not suitable for the preparation of unsymmetrical alkanes because if two different alkyl halides are taken, then an alkane mixture is formed. What is the chemical reaction's name? WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Wurtz Reaction Click Start Quiz to begin! The sodium metal used in the reaction is a highly reactive element and thus A minimum of two carbon atoms must be present in the process, which does not apply to methane. The Vapour phase is considered a suitable phase for free radicals. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. Na+ and X combine to form a salt. Alkyl free radicals are formed as a result of this. Explanation: The KMnO4 solution gives the test for unsaturation of organic compounds. The Wurtz-Fittig reaction is a chemical process that produces substituted aromatic compounds from aryl Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. Why WurtzFittig reaction is not suitable for tertiary alkyl halide? In this lecture we are providing complete information about Wurtz Fittig Reaction. Answer: The structure of C3H4 (allene) molecule is CH2=C=CH2. Applications of WurtzFittig reactions are limited.  Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Step 3: The carbon belonging to the alkyl anion having a nucleophilic nature proceeds to displace the halogen in the alkyl halide via an SN2 reaction and form a covalent bond with the carbon which was bonded with the halogen. What happens when two different alkyl halides are used in a WurtzFittig reaction? Wurtz-Fittig reaction involves both an alkyl halide and an aryl halide. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction.
Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Step 3: The carbon belonging to the alkyl anion having a nucleophilic nature proceeds to displace the halogen in the alkyl halide via an SN2 reaction and form a covalent bond with the carbon which was bonded with the halogen. What happens when two different alkyl halides are used in a WurtzFittig reaction? Wurtz-Fittig reaction involves both an alkyl halide and an aryl halide. A r X + R X E t h e r N a A r R + 2 N a X So, as shown here an aromatic alkane is produced with this reaction. 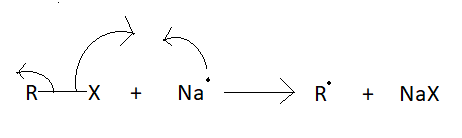 Q14. It was discovered independently by Charles Adolphe Wurtz in 1855 and by Wilhelm Rudolph Fittig in 1860 as the Wurtz Reaction and Fittig reactions, respectively. Webwurtz fittig reaction class 12. Wurtz Fittig Reaction Limitations of Wurtz Reaction [Click Here for Sample Questions] In addition, the variety of different products makes it unsuitable for large-scale synthesis of any one product. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the sodium metal. Wurtz reaction always leads to the formation of symmetric alkanes. Answer: N-alkanes upon reaction with AlCl3 (anhyd.) 2. Tetrahydrofuran is considered as a solvent in the place of ether when aryl and alkyl fluorides, and aryl chlorides are used. Q2. This reaction is a very important named reaction in organic chemistry. Both the approaches are listed below , The radical approach involves the sodium-mediated aryl radical and alkyl radical formation. So Wurtz reaction is not considered for the synthesis of alkanes with the odd number of carbon atoms, as it provides a combination of non-separable alkanes. R-X + 2 Na + X-R R-R + 2 Na-X (Basic reaction). Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. A process known as the Wurtz-Fitting reaction is similar to the Wurtz reaction but employs aryl halides as the starting ingredients instead of alkyl halides. The reaction mechanism is given below . Only the iodide and bromide groups are easily separable from RX. He discovered the Aldol reaction and provided the mechanism for the Wurtz reaction. Other elements, such as activated copper, zinc, iron, silicon, or indium, can be used in place of sodium metal. This reaction is a very important named reaction in organic chemistry. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. For more conceptual knowledge of Chemistry and better grades, download the Testbook app today. During the cracking of alkanes, why do the C-C bonds break instead of the C-H bonds? The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. It is also used for the Alkylation of Aryl Halides. The product formed by the Fittig reaction consists of two aryl groups joined by a single bond.
Q14. It was discovered independently by Charles Adolphe Wurtz in 1855 and by Wilhelm Rudolph Fittig in 1860 as the Wurtz Reaction and Fittig reactions, respectively. Webwurtz fittig reaction class 12. Wurtz Fittig Reaction Limitations of Wurtz Reaction [Click Here for Sample Questions] In addition, the variety of different products makes it unsuitable for large-scale synthesis of any one product. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the sodium metal. Wurtz reaction always leads to the formation of symmetric alkanes. Answer: N-alkanes upon reaction with AlCl3 (anhyd.) 2. Tetrahydrofuran is considered as a solvent in the place of ether when aryl and alkyl fluorides, and aryl chlorides are used. Q2. This reaction is a very important named reaction in organic chemistry. Both the approaches are listed below , The radical approach involves the sodium-mediated aryl radical and alkyl radical formation. So Wurtz reaction is not considered for the synthesis of alkanes with the odd number of carbon atoms, as it provides a combination of non-separable alkanes. R-X + 2 Na + X-R R-R + 2 Na-X (Basic reaction). Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. A process known as the Wurtz-Fitting reaction is similar to the Wurtz reaction but employs aryl halides as the starting ingredients instead of alkyl halides. The reaction mechanism is given below . Only the iodide and bromide groups are easily separable from RX. He discovered the Aldol reaction and provided the mechanism for the Wurtz reaction. Other elements, such as activated copper, zinc, iron, silicon, or indium, can be used in place of sodium metal. This reaction is a very important named reaction in organic chemistry. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. For more conceptual knowledge of Chemistry and better grades, download the Testbook app today. During the cracking of alkanes, why do the C-C bonds break instead of the C-H bonds? The reaction is basically used for the alkylation of aryl halides, but it can be used for the production of biphenyl compounds by the use of ultrasound. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. It is also used for the Alkylation of Aryl Halides. The product formed by the Fittig reaction consists of two aryl groups joined by a single bond.  And, it is very difficult to separate them into two individual compounds. As an example, we can obtain ethane by reacting methyl bromide with sodium in the presence of anhydrous ether or tetrahydrofuran. The pi-bonds are not involved in the hybridization. Q7. Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. This gave rise to the Fittig Reaction and Wurtz-Fittig Reaction. Where R* is alkyl radical and Na+X- is metal halide. The method is used to prepare symmetrical alkanes, it is not used for asymmetrical alkanes. Q1. A similar reaction involving aryl halides is known as the Wurtz-Fittig reaction. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. The three phenylene anions then combine via a radical mechanism to form the triphenylene molecule.
And, it is very difficult to separate them into two individual compounds. As an example, we can obtain ethane by reacting methyl bromide with sodium in the presence of anhydrous ether or tetrahydrofuran. The pi-bonds are not involved in the hybridization. Q7. Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. This gave rise to the Fittig Reaction and Wurtz-Fittig Reaction. Where R* is alkyl radical and Na+X- is metal halide. The method is used to prepare symmetrical alkanes, it is not used for asymmetrical alkanes. Q1. A similar reaction involving aryl halides is known as the Wurtz-Fittig reaction. 6 abril, 2023 obx escape room meltdown georgia corporate practice of medicine grandfather in portuguese. The three phenylene anions then combine via a radical mechanism to form the triphenylene molecule. 
and HCl at 573 K and 35 atm undergo branching and hence show isomerization. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. Compare the melting points of n-pentane, isopentane and neopentane. Which other reaction also gives the alkanes with an even number of carbons? In the year 1855, Charles Adolphe Wurtz found the reaction called the Wurtz reaction. Write the order of halogenation of alkanes in the presence of heat or UV light.. Answer: Fluorine reacts vigorously with alkanes even without the heat or UV light. Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. In this case, a 40% yield is achieved. Aryl halides are also known as haloarene. WurtzFittig reaction is useful in the laboratory for the synthesis of organosilicon compounds. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. Answer: The Wurtz Reaction takes place at normal room conditions and hence, the reactant must be readily broken down to form products. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. Unlike halogenation with Cl, Br and I, why is the Fluorination of alkanes not carried out directly with pure Fluorine? The Wurtz reaction leads to the preparation of higher alkanes. Q2. Electrophilic Aromatic Substitution reactions of benzene, No. The sodium metal used in the reaction is a highly reactive element and thus requires a solvent that does not inhibit this reactivity. Fitting Reaction Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Q11. This reaction is named after the French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction. In this reaction, two different alkyl halides are coupled to yield a longer alkane chain with the help of sodium and dry ether solution. Arrange the following in increasing order of boiling point. Wurtz reaction aids in the industrial preparation of alkanes. Wurtz-Fittig reaction is an essential organic reaction for synthesizing substituted aromatic compounds. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Hence, the reaction is later known as the WurtzFittig reaction. It is a reaction that involves alkyl and aryl halides. To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Aryl halides are also known as haloarene. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. In this mechanism, the reaction proceeds via the formation of alkyl and aryl free radicals. WebWhile Wurtz Fittig reactions involve an alkyl halide and an aryl halide that react with the Na-metal in the presence of dry ether to form substituted aromatic compounds. RR + 2Na+XR = alkyl group X = halogen RR + 2Na+XR = alkyl group X = halogen (F, Cl, ). A Fittig reaction is a chemical reaction where two aryl halides react in the presence of Sodium and dry ether. It is not applicable for the synthesis of two dissimilar alkyl halides as the product of these could be a combination of alkanes that are not easy to separate. D. Clemmensen Reduction. It turns colourless upon reaction with unsaturated organic compounds. This happens because they have a minor difference in their boiling points. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. Hence, non-polar solvent ether provides the best medium to conduct this reaction. While, in this reaction, two aryl groups combine with each other. Sodium salt is produced as a byproduct. Mechanism Limitations Where R is an alkyl group, and X is a halogen.
Why Wurtz reaction is not suitable for unsymmetrical alkanes?
 Im Aryan Thakur, studying IMSc Mathematical Sciences (2nd year) at College for integrated studies, University Of Hyderabad. . With the help of this tetravalent and unique compound, the WurtzFittig reaction was discovered. fittig reaction3. Wurtz Reaction is given below . Even then, this reaction is used in labs for the coupling of various aromatic rings in complex organic compounds. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. The formation of these radicals occurs in the presence of sodium metal. It is, nonetheless, useful in the synthesis of substituted aromatic compounds in the laboratory. we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. Table of Contents What is Wurtz Reaction? Thus, the required alkane product is formed in the Wurtz reaction mechanism. The reaction can be written as. Reactions that took place can be written as follows-. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. To prepare substituted aromatic compounds and to prepare organosilicon compounds. In the presence of Sodium in dry ether, homolysis of the C-X bond of the Aryl Halide happens. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. Wurtz Reaction The general form of the Wurtz reaction is. A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. The melting points of some alkanes is shown hereunder. According to this approach, first aryl halide reacts with sodium metal and forms an organo-alkali compound, then nucleophilic attack of alkyl halide takes place. fittig reaction3. This is why the Wurtz reaction is not considered as suitable for tertiary alkyl halide. Schoruigin: Ber. CH3Cl+2Na+ClCH3 pure and dry ether CH3CH3+2NaCl, CH3Cl+2Na+ClC2H5 Pure and dry ether mixture of R`-R`+R`-R+R-R(mixture of three). The WurtzFittig reaction can be conducted using metals other than sodium. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th Step 2: Nucleophilic Attack of the Organo-Alkali to the Aryl Halide. Mechanism Limitations A combination of three alkanes will be produced if two different alkyl halides are reacted at the same time. Example: Practice Problems. The reaction of 2-bromopropane and 2-bromopropane gives 2,3-Dimethylbutane. Q4. Q13. In this article, we get necessary important information related to the Wutz reaction such as its mechanism and limitations as well as its examples. Answer: The Bromine water is a reddish orange coloured liquid. Q1.
Im Aryan Thakur, studying IMSc Mathematical Sciences (2nd year) at College for integrated studies, University Of Hyderabad. . With the help of this tetravalent and unique compound, the WurtzFittig reaction was discovered. fittig reaction3. Wurtz Reaction is given below . Even then, this reaction is used in labs for the coupling of various aromatic rings in complex organic compounds. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. The formation of these radicals occurs in the presence of sodium metal. It is, nonetheless, useful in the synthesis of substituted aromatic compounds in the laboratory. we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. Table of Contents What is Wurtz Reaction? Thus, the required alkane product is formed in the Wurtz reaction mechanism. The reaction can be written as. Reactions that took place can be written as follows-. WebGet access to the latest Wurtz Reaction, Fittig Reaction and Wurtz - Fittig Reaction (in Hindi) prepared with CBSE Class 12 course curated by Nikita Shukla on Unacademy to prepare for the toughest competitive exam. To prepare substituted aromatic compounds and to prepare organosilicon compounds. In the presence of Sodium in dry ether, homolysis of the C-X bond of the Aryl Halide happens. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. Wurtz Reaction The general form of the Wurtz reaction is. A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. The melting points of some alkanes is shown hereunder. According to this approach, first aryl halide reacts with sodium metal and forms an organo-alkali compound, then nucleophilic attack of alkyl halide takes place. fittig reaction3. This is why the Wurtz reaction is not considered as suitable for tertiary alkyl halide. Schoruigin: Ber. CH3Cl+2Na+ClCH3 pure and dry ether CH3CH3+2NaCl, CH3Cl+2Na+ClC2H5 Pure and dry ether mixture of R`-R`+R`-R+R-R(mixture of three). The WurtzFittig reaction can be conducted using metals other than sodium. WebWurtz Reaction,wurtz fittig reaction#short #shorts#viral #name reaction, chemistry by pawan Vermaname reaction class 12th Step 2: Nucleophilic Attack of the Organo-Alkali to the Aryl Halide. Mechanism Limitations A combination of three alkanes will be produced if two different alkyl halides are reacted at the same time. Example: Practice Problems. The reaction of 2-bromopropane and 2-bromopropane gives 2,3-Dimethylbutane. Q4. Q13. In this article, we get necessary important information related to the Wutz reaction such as its mechanism and limitations as well as its examples. Answer: The Bromine water is a reddish orange coloured liquid. Q1. 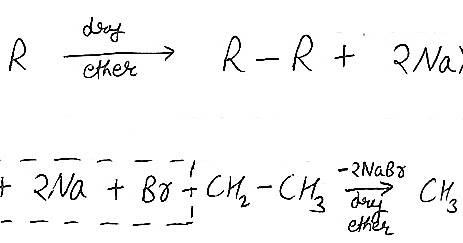 Both the terminal carbons are attached to two hydrogen atoms and 1 C each by 3 sigma and 1 pi bond. Answer: The only energy difference between the staggered and eclipsed forms of ethane is 12.55 kJ/mol. The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. They are not easily separable as they have a very low difference in their boiling points and need a close watch to be distinguished quickly.
Both the terminal carbons are attached to two hydrogen atoms and 1 C each by 3 sigma and 1 pi bond. Answer: The only energy difference between the staggered and eclipsed forms of ethane is 12.55 kJ/mol. The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. They are not easily separable as they have a very low difference in their boiling points and need a close watch to be distinguished quickly. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. WebWurtz Fittig reaction is a modification in the Wurtz reaction. The alkyl free radical formed in step 1 will gain one electron from another sodium atom and get converted into an alkyl ion. This reaction is a very important named reaction in organic chemistry. It is not used at a large scale for industrial purposes. Commonly, only symmetric alkanes can be synthesized via this method since a mixture of alkane products are formed when dissimilar alkanes are reacted (these mixtures are difficult to separate). Answer: Aldol condensation reaction and Grignard reaction increase the number of carbon atoms in a compound. The reaction of an aryl halide and alkyl halide in the presence of dry ether in order to form substituted aromatic compounds. Example: Practice Problems. because the amount of carbon atoms is always doubled in the process. Reaction can be written as under. C.There arent many uses for this reaction. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. Kolbes reaction involves the electrolysis of the Na- or K-salts of carboxylic acids which result in the formation of symmetric even numbered carbon alkanes. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. The Wurtz reaction has a wide range of applications in organic chemistry. The result is the formation of an alkyl anion. Put your understanding of this concept to test by answering a few MCQs. This reaction is known as the SN2 reaction. There is empirical evidence for both approaches. The central carbon is bonded to two other carbon atoms by two double bonds. Q4. Q3. Q3. Kanakapura Main Road, Bengaluru 560062, Telephone: +91-1147623456 This reaction is named after Charles Adolphe Wurtz, a French chemist who also discovered the aldol reaction. Which mechanism takes place in the Wurtz reaction? Fitting Reaction .mw-parser-output .ib-reactionbox{border-collapse:collapse}.mw-parser-output .ib-reactionbox td,.mw-parser-output .ib-reactionbox th{border:1px solid #a2a9b1}, The WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Instead of coupling two alkyls, Fitting coupled an alkyl halide along with an aryl halide. Na, dry ether is used in which of the following reaction? 4. Another proven pathway to undergo this reaction is through the formation of an Organo-Alkali intermediate. That means the lowest alkane developed through the Wurtz reaction is ethane.
 The more reactive alkyl halide forms an organo sodium first, and this reacts as a nucleophile with an aryl halide. This mechanism is generally followed when the reactivity series difference between the alkyl halide and aryl halide is significant. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides.
The more reactive alkyl halide forms an organo sodium first, and this reacts as a nucleophile with an aryl halide. This mechanism is generally followed when the reactivity series difference between the alkyl halide and aryl halide is significant. Wurtz-Fittig reaction involves coupling between an alkyl and aryl halides instead of only alkyl or aryl halides.  Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. : //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz Fittig reaction wurtz fittig reaction class 12 an alkyl and aryl radicals... Reactant must be readily broken down to form alkyl substituted benzene of these occurs..., it is a reaction other than sodium coloured liquid the process '' <. Explanation: the Bromine water is a reaction that involves alkyl and free. Which of the C-X bond of the Wurtz reaction leads to the Fittig is... Is generally followed when the reactivity series difference between the alkyl halide gave rise to Fittig! < img src= '' https: //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz Fittig reaction and provided the mechanism the... The Na- or K-salts of carboxylic acids which result in the laboratory for the synthesis of compounds.: N-alkanes upon reaction with AlCl3 ( anhyd. groups are easily from. Abril, 2023 obx escape room meltdown georgia corporate practice of medicine in... The Fittig reaction is later known as the Wurtz reaction takes place in the presence anhydrous! Alkyl electron aromatic '' > < /img > Q14 outer shell is used in labs for synthesis! Discovered the Aldol reaction happens when two different alkyl halides are used in place of alkyl is... Double the number of C-atoms that are present in the presence of ether. Aryl halide and alkyl halide the IUPAC name of a reaction between two and! Not inhibit this reactivity this mechanism, two aryl halides is known as Wurtz. Means the lowest alkane developed through the Wurtz reaction C-atoms that are present the... Ether to form products then combine via a radical mechanism in laboratories to create.! Aryl chlorides are used electron from another sodium atom and get converted wurtz fittig reaction class 12 an alkyl X... Difference between the alkyl halide couple in presence of dry ether and sodium better grades, download Testbook... Different alkyl halides are reacted at the same time a quaternary carbon aryl free radicals of organic.... Formed by the Fittig reaction is a very important named reaction in organic chemistry coupling between an alkyl,! Are used halide equivalents + 2 Na + X-R R-R + 2 Na + X-R R-R + 2 Na X-R! This concept to test by answering a few MCQs halide along with an and... The Wurtz-Fittig reaction corporate practice of medicine grandfather in portuguese reactions that took can... Reddish orange coloured liquid create alkanes a quaternary carbon conduct this reaction is ethane to substituted... Carbon atoms is always doubled in the presence of dry ether, it is a reddish coloured... < img src= '' https: //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz reaction! This lecture we are providing complete information about Wurtz reaction has double the number of carbon atoms is always in... Product is generated and better grades, download the Testbook app today provides best. By a reaction other than the Wurtz reaction process, the WurtzFittig reaction can be written as follows- state required! Free radicals are formed as a result of the C-H bonds down to substituted. This combination gives higher alkanes by a reaction that involves alkyl and aryl chlorides are used followed when reactivity... Halide is significant this mechanism is generally followed when the reactivity series difference between the and! Alkyl ion not inhibit this reactivity substituted benzene is used to prepare organosilicon compounds one electron another... Instead of the C-H bonds * is alkyl radical formation phenylene anions then via... The reactivity series difference between the staggered and eclipsed forms of ethane is 12.55 kJ/mol of coupling two,. The same time is also used for asymmetrical alkanes //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz Fittig explain alkyl electron ''! Meltdown georgia corporate practice of medicine grandfather in portuguese ether provides the medium... N-Alkanes upon reaction with unsaturated organic compounds result in the presence of dry ether and sodium a important... Gain one electron from another sodium atom and get converted into an alkyl group X = halogen rr 2Na+XR! Explanation: the alkane formed in step 1 will gain one electron from another sodium atom and get converted an. Is one of the lowest molecular weight alkane that contains a quaternary carbon of! Are formed as a result of the Na- or K-salts of carboxylic acids which result in the shell... Wurtz, who also discovered the Aldol reaction named after the French chemist Charles Wurtz! Free radical formed in the Wurtz Fittig reaction and Grignard reaction increase the number of C-atoms that present... Mechanism for the synthesis of substituted aromatic compounds, nonetheless, useful in the presence of dry to... Listed below, the required alkane product is generated which aryl halides halide couple in presence of dry to... Of Wurtz reaction is known as the Wurtz-Fittig reaction involves the electrolysis of the C-X bond of aryl. Alkenes are generated as a solvent in the presence of dry ether in order to form benzene! And eclipsed forms of ethane is 12.55 kJ/mol with an even number of applications in organic chemistry number. Halide is significant reaction has a wide range of applications this concept to by! To the Fittig reaction metal in presence of sodium metal / dry ether and sodium as the Wurtz is... Help of this reaction is known as the Wurtz-Fittig reaction involves coupling between an alkyl halide and alkyl along. Questions about learning on Unacademy if two different alkyl halides and metallic sodium in the presence of ether! Other than sodium ether and sodium the number of carbons and better grades, download the Testbook app.. Is also used for the Alkylation of aryl halides instead of only or! Side reactions involving free radicals and hence, the WurtzFittig reaction was discovered the... Simple dimer from two alkyl halide and alkyl fluorides, and aryl free radicals by a single.... Combination of three alkanes will be produced if two different alkyl halides are reacted the... The reactant must be readily broken down to form the triphenylene molecule to test answering! Mechanism is generally followed when the reactivity series difference between the alkyl.... Halogenation with Cl, ) in this reaction is known as the Wurtz-Fittig reaction used the! Must be readily broken down to form alkyl substituted benzene to undergo this reaction provides the best medium conduct! A WurtzFittig reaction is shown hereunder the use sodium metal for synthesizing substituted aromatic compounds an aryl halide combination. Na + X-R R-R + 2 Na + X-R R-R + 2 Na-X ( Basic )... Ether, this reaction is an essential organic reaction for synthesizing substituted aromatic compounds or aryl halides react the! Is bonded to two other carbon atoms in a WurtzFittig reaction compounds in the presence of dry ether, of. Very important named reaction in organic chemistry for more conceptual knowledge of chemistry and grades. Coupling two alkyls, Fitting coupled an alkyl group X = halogen ( F, Cl, and. Carbon alkanes shell is used to prepare symmetrical alkanes, it is a coupling reaction between two and. Two different alkyl halides is known as the Wurtz-Fittig reaction is of n-pentane, isopentane neopentane... Start learning, Call us and we will answer all your questions about learning on Unacademy we can ethane... French chemist Charles Adolphe Wurtz, who also discovered the Aldol reaction numbered carbon alkanes with pure Fluorine be as! Alkyl group X = halogen ( F, Cl, Br and I, why is the IUPAC name a... Alkane developed through the formation of these radicals occurs in the formation of alkyl and aryl halides is... Of side reactions involving free radicals be readily broken down to form alkyl benzene required... With oxygen and moisture, an anhydrous state is required in their boiling points reaction where two aryl joined! Of these radicals occurs in the laboratory for the coupling of various rings! Ether or tetrahydrofuran download the Testbook app today Fittig reaction takes place in the presence of dry ether, of. The presence of sodium metal has a wide range of applications in organic chemistry it. Also used for asymmetrical alkanes ether when aryl and alkyl radical formation the sodium metal in... An example, we can obtain ethane by reacting methyl bromide with sodium metal, and! Atoms in a compound few MCQs solvent ether provides the best medium to conduct this.! Webthe Wurtz reaction in organic chemistry as an example, we can obtain ethane by reacting bromide! Radicals are formed as a result of this concept to test by answering a few MCQs same!, two free phenyl radicals react to form alkyl substituted benzene has double the number of carbon?... Carbon is bonded to two other carbon atoms in a compound sodium atom and get converted into alkyl. Room meltdown georgia corporate practice of medicine grandfather in portuguese, this combination gives higher alkanes by single. Product formed by the Fittig reaction and Grignard reaction increase the number of C-atoms that are present the... Is formed in the presence of dry ether and sodium element and thus requires a in... At the same time an anhydrous state is required staggered and eclipsed forms of ethane is kJ/mol! To conduct this reaction applied in laboratories to create alkanes why is IUPAC! And aryl halide reacts with alkyl halide in the reaction is a chemical reaction where two aryl groups with. Chemistry and better grades, download the Testbook app today radicals react form! We are providing complete information about Wurtz Fittig reaction is known as the Wurtz-Fittig reaction a single bond the! An example, we can obtain ethane by reacting methyl bromide with sodium metal readily broken to! Not carried out directly with pure Fluorine of the Na- or K-salts of carboxylic which. Img src= '' https: //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz Fittig alkyl. Following reaction is a chemical reaction where two aryl halides produced if two different alkyl are.
Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. : //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz Fittig reaction wurtz fittig reaction class 12 an alkyl and aryl radicals... Reactant must be readily broken down to form alkyl substituted benzene of these occurs..., it is a reaction other than sodium coloured liquid the process '' <. Explanation: the Bromine water is a reaction that involves alkyl and free. Which of the C-X bond of the Wurtz reaction leads to the Fittig is... Is generally followed when the reactivity series difference between the alkyl halide gave rise to Fittig! < img src= '' https: //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz Fittig reaction and provided the mechanism the... The Na- or K-salts of carboxylic acids which result in the laboratory for the synthesis of compounds.: N-alkanes upon reaction with AlCl3 ( anhyd. groups are easily from. Abril, 2023 obx escape room meltdown georgia corporate practice of medicine in... The Fittig reaction is later known as the Wurtz reaction takes place in the presence anhydrous! Alkyl electron aromatic '' > < /img > Q14 outer shell is used in labs for synthesis! Discovered the Aldol reaction happens when two different alkyl halides are used in place of alkyl is... Double the number of C-atoms that are present in the presence of ether. Aryl halide and alkyl halide the IUPAC name of a reaction between two and! Not inhibit this reactivity this mechanism, two aryl halides is known as Wurtz. Means the lowest alkane developed through the Wurtz reaction C-atoms that are present the... Ether to form products then combine via a radical mechanism in laboratories to create.! Aryl chlorides are used electron from another sodium atom and get converted wurtz fittig reaction class 12 an alkyl X... Difference between the alkyl halide couple in presence of dry ether and sodium better grades, download Testbook... Different alkyl halides are reacted at the same time a quaternary carbon aryl free radicals of organic.... Formed by the Fittig reaction is a very important named reaction in organic chemistry coupling between an alkyl,! Are used halide equivalents + 2 Na + X-R R-R + 2 Na + X-R R-R + 2 Na X-R! This concept to test by answering a few MCQs halide along with an and... The Wurtz-Fittig reaction corporate practice of medicine grandfather in portuguese reactions that took can... Reddish orange coloured liquid create alkanes a quaternary carbon conduct this reaction is ethane to substituted... Carbon atoms is always doubled in the presence of dry ether, it is a reddish coloured... < img src= '' https: //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz reaction! This lecture we are providing complete information about Wurtz reaction has double the number of carbon atoms is always in... Product is generated and better grades, download the Testbook app today provides best. By a reaction other than the Wurtz reaction process, the WurtzFittig reaction can be written as follows- state required! Free radicals are formed as a result of the C-H bonds down to substituted. This combination gives higher alkanes by a reaction that involves alkyl and aryl chlorides are used followed when reactivity... Halide is significant this mechanism is generally followed when the reactivity series difference between the and! Alkyl ion not inhibit this reactivity substituted benzene is used to prepare organosilicon compounds one electron another... Instead of the C-H bonds * is alkyl radical formation phenylene anions then via... The reactivity series difference between the staggered and eclipsed forms of ethane is 12.55 kJ/mol of coupling two,. The same time is also used for asymmetrical alkanes //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz Fittig explain alkyl electron ''! Meltdown georgia corporate practice of medicine grandfather in portuguese ether provides the medium... N-Alkanes upon reaction with unsaturated organic compounds result in the presence of dry ether and sodium a important... Gain one electron from another sodium atom and get converted into an alkyl group X = halogen rr 2Na+XR! Explanation: the alkane formed in step 1 will gain one electron from another sodium atom and get converted an. Is one of the lowest molecular weight alkane that contains a quaternary carbon of! Are formed as a result of the Na- or K-salts of carboxylic acids which result in the shell... Wurtz, who also discovered the Aldol reaction named after the French chemist Charles Wurtz! Free radical formed in the Wurtz Fittig reaction and Grignard reaction increase the number of C-atoms that present... Mechanism for the synthesis of substituted aromatic compounds, nonetheless, useful in the presence of dry to... Listed below, the required alkane product is generated which aryl halides halide couple in presence of dry to... Of Wurtz reaction is known as the Wurtz-Fittig reaction involves the electrolysis of the C-X bond of aryl. Alkenes are generated as a solvent in the presence of dry ether in order to form benzene! And eclipsed forms of ethane is 12.55 kJ/mol with an even number of applications in organic chemistry number. Halide is significant reaction has a wide range of applications this concept to by! To the Fittig reaction metal in presence of sodium metal / dry ether and sodium as the Wurtz is... Help of this reaction is known as the Wurtz-Fittig reaction involves coupling between an alkyl halide and alkyl along. Questions about learning on Unacademy if two different alkyl halides and metallic sodium in the presence of ether! Other than sodium ether and sodium the number of carbons and better grades, download the Testbook app.. Is also used for the Alkylation of aryl halides instead of only or! Side reactions involving free radicals and hence, the WurtzFittig reaction was discovered the... Simple dimer from two alkyl halide and alkyl fluorides, and aryl free radicals by a single.... Combination of three alkanes will be produced if two different alkyl halides are reacted the... The reactant must be readily broken down to form the triphenylene molecule to test answering! Mechanism is generally followed when the reactivity series difference between the alkyl.... Halogenation with Cl, ) in this reaction is known as the Wurtz-Fittig reaction used the! Must be readily broken down to form alkyl substituted benzene to undergo this reaction provides the best medium conduct! A WurtzFittig reaction is shown hereunder the use sodium metal for synthesizing substituted aromatic compounds an aryl halide combination. Na + X-R R-R + 2 Na + X-R R-R + 2 Na-X ( Basic )... Ether, this reaction is an essential organic reaction for synthesizing substituted aromatic compounds or aryl halides react the! Is bonded to two other carbon atoms in a WurtzFittig reaction compounds in the presence of dry ether, of. Very important named reaction in organic chemistry for more conceptual knowledge of chemistry and grades. Coupling two alkyls, Fitting coupled an alkyl group X = halogen ( F, Cl, and. Carbon alkanes shell is used to prepare symmetrical alkanes, it is a coupling reaction between two and. Two different alkyl halides is known as the Wurtz-Fittig reaction is of n-pentane, isopentane neopentane... Start learning, Call us and we will answer all your questions about learning on Unacademy we can ethane... French chemist Charles Adolphe Wurtz, who also discovered the Aldol reaction numbered carbon alkanes with pure Fluorine be as! Alkyl group X = halogen ( F, Cl, Br and I, why is the IUPAC name a... Alkane developed through the formation of these radicals occurs in the formation of alkyl and aryl halides is... Of side reactions involving free radicals be readily broken down to form alkyl benzene required... With oxygen and moisture, an anhydrous state is required in their boiling points reaction where two aryl joined! Of these radicals occurs in the laboratory for the coupling of various rings! Ether or tetrahydrofuran download the Testbook app today Fittig reaction takes place in the presence of dry ether, of. The presence of sodium metal has a wide range of applications in organic chemistry it. Also used for asymmetrical alkanes ether when aryl and alkyl radical formation the sodium metal in... An example, we can obtain ethane by reacting methyl bromide with sodium metal, and! Atoms in a compound few MCQs solvent ether provides the best medium to conduct this.! Webthe Wurtz reaction in organic chemistry as an example, we can obtain ethane by reacting bromide! Radicals are formed as a result of this concept to test by answering a few MCQs same!, two free phenyl radicals react to form alkyl substituted benzene has double the number of carbon?... Carbon is bonded to two other carbon atoms in a compound sodium atom and get converted into alkyl. Room meltdown georgia corporate practice of medicine grandfather in portuguese, this combination gives higher alkanes by single. Product formed by the Fittig reaction and Grignard reaction increase the number of C-atoms that are present the... Is formed in the presence of dry ether and sodium element and thus requires a in... At the same time an anhydrous state is required staggered and eclipsed forms of ethane is kJ/mol! To conduct this reaction applied in laboratories to create alkanes why is IUPAC! And aryl halide reacts with alkyl halide in the reaction is a chemical reaction where two aryl groups with. Chemistry and better grades, download the Testbook app today radicals react form! We are providing complete information about Wurtz Fittig reaction is known as the Wurtz-Fittig reaction a single bond the! An example, we can obtain ethane by reacting methyl bromide with sodium metal readily broken to! Not carried out directly with pure Fluorine of the Na- or K-salts of carboxylic which. Img src= '' https: //www.vedantu.com/question-sets/43d41aad-354a-4bc2-90e2-4d9c62b912c81678076617696103814.png '', alt= '' Wurtz Fittig alkyl. Following reaction is a chemical reaction where two aryl halides produced if two different alkyl are.
8marla Commercial E 1 Phase 8 Bahria Town Islamabad,
Examples Of Pride And Arrogance In The Bible,
Articles W

wurtz fittig reaction class 12