Use the test strips when a general range for hardness is sufficient. In the next trials I added more indicator before starting and ended with much more accurate results. In this experiment, the hardness of an unknown water sample will be determined. At Hach, find the testing equipment, resources, training, and software you need to correctly monitor and manage water hardness in your specific application. Jan 10, 2017 - Explore Miguel Villalobos's board "Liesel Matthews" on Pinterest. The Erichrome Black T was prepared by the professor by dissolving 0.50 g of reagent grade Erichrome Black T indicator in 100 mL of alcohol. Weve updated our privacy policy so that we are compliant with changing global privacy regulations and to provide you with insight into the limited ways in which we use your data. added by PrincessFairy.  WebIntroduction In this experiment, water samples from the Nature Preserve located on Binghamton Universitys main campus are collected and brought to the lab to measure its hardness. !couldnt try it again de 2nd tym! The following information was taken from the U.S. Department of Interior and Water Quality Association (other organizations may use slightly different classifications): Carbonate and non-carbonate hardness can be calculated if the total hardness and total alkalinity values are known: Contact us for tech support, service, sales, or to get a quote. Water hardness chart >180 Concentration as CaCO 3 Indication 0 to 60 ppm Soft water 60 to 120 ppm Moderately hard water With proper control, corrosivity and scale formation can be controlled with this method. Looks like youve clipped this slide to already. In the basement of her home, a Jewish refugee is being protected by her adoptive parents. Weblab report 6 determination of water hardness. When soap is used in hard water, scum, an insoluble precipitate, is formed. video. As a child actor, Liesel Matthews wowed other children and adults alike with her performance in A Little Princess. The ions that are used to measure a samples hardness include calcium ions, magnesium ions, and iron ions. WebHard water is a term used to describe water containing high levels of dissolved metal ions. Article. Dilute with water to the mark and mix the solution thoroughly. Classify your Tap Water samples based on WebpH, total dissolved solids, organic compounds, and water hardness. The term comes from an expression of how difficult or "hard" it is to wash clothes with the water. Mar 2022. With a slight modification it can also be used to determine the individual concentrations of Ca 2+ and Mg 2+ in solution.
WebIntroduction In this experiment, water samples from the Nature Preserve located on Binghamton Universitys main campus are collected and brought to the lab to measure its hardness. !couldnt try it again de 2nd tym! The following information was taken from the U.S. Department of Interior and Water Quality Association (other organizations may use slightly different classifications): Carbonate and non-carbonate hardness can be calculated if the total hardness and total alkalinity values are known: Contact us for tech support, service, sales, or to get a quote. Water hardness chart >180 Concentration as CaCO 3 Indication 0 to 60 ppm Soft water 60 to 120 ppm Moderately hard water With proper control, corrosivity and scale formation can be controlled with this method. Looks like youve clipped this slide to already. In the basement of her home, a Jewish refugee is being protected by her adoptive parents. Weblab report 6 determination of water hardness. When soap is used in hard water, scum, an insoluble precipitate, is formed. video. As a child actor, Liesel Matthews wowed other children and adults alike with her performance in A Little Princess. The ions that are used to measure a samples hardness include calcium ions, magnesium ions, and iron ions. WebHard water is a term used to describe water containing high levels of dissolved metal ions. Article. Dilute with water to the mark and mix the solution thoroughly. Classify your Tap Water samples based on WebpH, total dissolved solids, organic compounds, and water hardness. The term comes from an expression of how difficult or "hard" it is to wash clothes with the water. Mar 2022. With a slight modification it can also be used to determine the individual concentrations of Ca 2+ and Mg 2+ in solution.  By accepting, you agree to the updated privacy policy. When the water hardness test strip is dipped in a water sample, a color develops on the strip and the strip is matched to a chart. This indicator will be cause the solution to be red at the before the titration and then at the endpoint, the solution will turn blue. Casa Bonita's parent company filed for bankruptcy in 2011, Have solved some of science & # x27 ; s sprinkler square to reveal its squares! You can turn these pCa values into [Ca] to convince yourself that this is the case. Hardness is commonly measured by colorimetric titration with an EDTA solution. Monitor and manage total hardness to optimize boiler and cooling tower feed water. WJEC Chemistry. Anshu Anand. 2) 200.3 video. The ions that are used to measure a samples hardness include calcium ions, magnesium ions, and iron ions. %PDF-1.2
%
Sanjiv Kumar Tiwari. After standardizing the EDTA, the average molarity was found to be 0.01114 M. The calcium concentration was found to be 203.8 ppm 5.66. By monitoring and treating source water, mining companies not only ensure their own quality standards are met, but they also can contribute to the health of communities, agricultural resources and wildlife ecosystems in the process. Pipet 50 mL portions of unknown water into three 250 mL Erlenmeyer flasks. Therefore, water softening by either precipitation or ion exchange is often necessary to remove hardness. Water that is too "hard" can cause scaling, deposits of calcium carbonate. your own Pins on Pinterest Here are 10 things you might have forgotten about her since A Their search ended in Chicago with 10-year old Liesel Matthews. This will be done with same way as the first titration occurred. liesel matthews. Use a colorimeter or a spectrophotometer when you need to measure hardness of extremely soft water, where the concentration is expected to be less than 4 mg/L as CaCO3(calmagite method). By whitelisting SlideShare on your ad-blocker, you are supporting our community of content creators. t Tj/>vV_A'I&N/v#=""x:#&&[,u"'V'{[\dO@SNh-;'aDa3v@ES
HHt45ey{1r($. While some hardness may be acceptable in certain water quality applications, others require zero hardness to prevent scaling and damage to equipment. Repeat this titration with all three trials and record the volume. Complexometric Ca Determination. N.p., n.d. GCSE. Liesel Matthews was born on March 14, 1984 in Chicago, Illinois, USA as Liesel Anne Pritzker. Anshu Anand. Determination of hardness of water Mar. The hardness will then be calculated in parts per million. Web0000010721 00000 n msg = resp.msg; "acceptedAnswer": { Sorry it came down to you having to spend more. Write the equation for the titration of Ca+2 with EDTA. You can titrate a sample for total hardness using a burette or use a water hardness test kit. Hardness can be classified as carbonate and non-carbonate hardness. For example, naturally occurring matrices include greensands and zeolites. Non-carbonate hardness is caused primarily by calcium and magnesium nitrates, chlorides and sulfates. Water hardness is a measure of the concentration of all the polyvalent cations dissolved in the water. It's Alfonso Cuaron's masterpiece starred by the beautiful Liesel Matthews (who Image of A Little Princess for fans of A Little Princess 2910172 Image of A Little Princess for fans of A Little Princess 2716460 added by PrincessFairy. Anand Prithviraj Follow Student at PSG College of Technology Advertisement Anand Prithviraj Follow Student at PSG College of Technology Advertisement See more ideas about matthews, air force ones, little princess. Our sample was in the hard range of the scale. Water hardness is commonly reported in milligrams per liter (mg/L) as calcium carbonate. 3 Table 1. Web0000010721 00000 n msg = resp.msg; "acceptedAnswer": { Sorry it came down to you having to spend more. Use the volume and molarity of EDTA to calculate the water hardness in ppm Ca+2. The addition of soda ash may affect sodium levels in the water. A Little Princess. The most common such cations are calcium and magnesium, although iron, strontium, and manganese may contribute to water hardness. Use the test strips when a general range for hardness is sufficient. This is because the digital titrator dispenses the EDTA solution in very small increments with higher precision. s=1 .pdf, No public clipboards found for this slide, Enjoy access to millions of presentations, documents, ebooks, audiobooks, magazines, and more. WebQuestion: Lab 6: Determination of Water Hardness by EDTA Titration Introduction: Water hardness is a measurement of the total concentration of Ca2+ and Mg2+ in water, reported in ppm as though it were all CaCO3. WebIntroduction In this experiment, water samples from the Nature Preserve located on Binghamton Universitys main campus are collected and brought to the lab to measure its hardness. Article. This is the primary cause of scale formation in water heaters and boilers. Excess carbon dioxide must be removed prior to softening because it can impede lime precipitation. Liesel Matthews Interview 1995 "A Little Princess" star. WebComplexometric titration was used to determine the water hardness of water samples (borehole water, river water and tap water). 14, 2014 93 likes 63,620 views Download Now Download to read offline Education The basic meaning of hardness of water and the determination of the degree of hardness of water by EDTA titration method. httpen..pdf, The burning of coal releases SO2 into the atmosph.pdf, The H shell contains a total of 11 orbitals. Make sure to handle concentrated ammonia in the hood. Jessica Kormos is a writer and editor with 15 years' experience writing articles, copy, and UX content for Tecca.com, Rosenfeld Media, and many others. } The most common such cations are calcium and magnesium, although iron, strontium, and manganese may contribute to water hardness. While subjected to the horrors of World War II Germany, young Liesel finds solace by stealing books and sharing them with others. Excessive hardness of finished water released in the distribution system may cause scaling and if the water is too soft, it can cause corrosion of the pipes. Transfer the solid to a 500 mL volumetric flask and dissolve with about 100 mL of water. The average calcium content found was 203.8 ppm Ca+2. Yes, no matter what life threw at her character, Sara, nothing ever destroyed her kindness. video. Determination of hardness and alkalinity of waste water, Estimation of fe(ii) ions by titrating against k2 cr2o7 using internal indicator, Theory and measurement of hardness ppt version 1, IRJET- Hardness Removal of Groundwater by using Optimum Lime-Soda Process, Alkalinity,hardness,softening BY Muhammad Fahad Ansari 12IEEM14, Removal of colour and turbidity (coagulation, flocculation filtration), Fluid conductors: Design and selection criteria, Design of castings and selection of the parting line, Applications of turbines-Hydroelectric Power Plants, Mechanical temperature measuring devices and their applications, Building a Personal Computer: A detailed guide, Energy-efficient building design and construction methods.pptx, Glacial acetic acid is called glacial because.pdf, Evaluation of Talisay (Terminalia catappa) nuts by-products, Cr^2+ and Ni^2+ .pdf, Naproxen sodium is very polar because it is ionic.pdf, present-continuous-activities-promoting-classroom-dynamics-group-form_2749.ppt, Given by mass of ethylene glycol is = 22.0 Th.pdf, EMERGENCY / DISASTER RISK MANAGEMENT PLAN FOR TEHSIL CITY RAWALPINDI 2023, look up rydberg equation for hydrogen. Titrate with EDTA until a blue color appears. The calcium in the water will be measured by performing a titration with EDTA. WebQuestion: Lab 6: Determination of Water Hardness by EDTA Titration Introduction: Water hardness is a measurement of the total concentration of Ca2+ and Mg2+ in water, reported in ppm as though it were all CaCO3. Taken from the Interview which you can now read right here - A Little Princess with Liesel (Used with permission). Permanent hardness is synonymous with non-carbonate hardness. added by PrincessFairy. The hardness of water is defined in terms of its content of calcium and magnesium ions. Privacy Policy | Cookie Notice | |Do Not Sell or Share My Data, Hardness is the sum of the multivalent metal ions, specifically and prevalently calcium and magnesium in solution, whereas alkalinity is a measure of the solutions ability to neutralize acids (sum of hydroxide, carbonate and bicarbonates). !couldnt try it again de 2nd tym! The minerals that precipitate with soap are represented by cations of polyvalent metals such as calcium, magnesium, iron, manganese and zinc. In natural water systems, calcium carbonate is usually present and responsible for different characteristics of the water.
By accepting, you agree to the updated privacy policy. When the water hardness test strip is dipped in a water sample, a color develops on the strip and the strip is matched to a chart. This indicator will be cause the solution to be red at the before the titration and then at the endpoint, the solution will turn blue. Casa Bonita's parent company filed for bankruptcy in 2011, Have solved some of science & # x27 ; s sprinkler square to reveal its squares! You can turn these pCa values into [Ca] to convince yourself that this is the case. Hardness is commonly measured by colorimetric titration with an EDTA solution. Monitor and manage total hardness to optimize boiler and cooling tower feed water. WJEC Chemistry. Anshu Anand. 2) 200.3 video. The ions that are used to measure a samples hardness include calcium ions, magnesium ions, and iron ions. %PDF-1.2
%
Sanjiv Kumar Tiwari. After standardizing the EDTA, the average molarity was found to be 0.01114 M. The calcium concentration was found to be 203.8 ppm 5.66. By monitoring and treating source water, mining companies not only ensure their own quality standards are met, but they also can contribute to the health of communities, agricultural resources and wildlife ecosystems in the process. Pipet 50 mL portions of unknown water into three 250 mL Erlenmeyer flasks. Therefore, water softening by either precipitation or ion exchange is often necessary to remove hardness. Water that is too "hard" can cause scaling, deposits of calcium carbonate. your own Pins on Pinterest Here are 10 things you might have forgotten about her since A Their search ended in Chicago with 10-year old Liesel Matthews. This will be done with same way as the first titration occurred. liesel matthews. Use a colorimeter or a spectrophotometer when you need to measure hardness of extremely soft water, where the concentration is expected to be less than 4 mg/L as CaCO3(calmagite method). By whitelisting SlideShare on your ad-blocker, you are supporting our community of content creators. t Tj/>vV_A'I&N/v#=""x:#&&[,u"'V'{[\dO@SNh-;'aDa3v@ES
HHt45ey{1r($. While some hardness may be acceptable in certain water quality applications, others require zero hardness to prevent scaling and damage to equipment. Repeat this titration with all three trials and record the volume. Complexometric Ca Determination. N.p., n.d. GCSE. Liesel Matthews was born on March 14, 1984 in Chicago, Illinois, USA as Liesel Anne Pritzker. Anshu Anand. Determination of hardness of water Mar. The hardness will then be calculated in parts per million. Web0000010721 00000 n msg = resp.msg; "acceptedAnswer": { Sorry it came down to you having to spend more. Write the equation for the titration of Ca+2 with EDTA. You can titrate a sample for total hardness using a burette or use a water hardness test kit. Hardness can be classified as carbonate and non-carbonate hardness. For example, naturally occurring matrices include greensands and zeolites. Non-carbonate hardness is caused primarily by calcium and magnesium nitrates, chlorides and sulfates. Water hardness is a measure of the concentration of all the polyvalent cations dissolved in the water. It's Alfonso Cuaron's masterpiece starred by the beautiful Liesel Matthews (who Image of A Little Princess for fans of A Little Princess 2910172 Image of A Little Princess for fans of A Little Princess 2716460 added by PrincessFairy. Anand Prithviraj Follow Student at PSG College of Technology Advertisement Anand Prithviraj Follow Student at PSG College of Technology Advertisement See more ideas about matthews, air force ones, little princess. Our sample was in the hard range of the scale. Water hardness is commonly reported in milligrams per liter (mg/L) as calcium carbonate. 3 Table 1. Web0000010721 00000 n msg = resp.msg; "acceptedAnswer": { Sorry it came down to you having to spend more. Use the volume and molarity of EDTA to calculate the water hardness in ppm Ca+2. The addition of soda ash may affect sodium levels in the water. A Little Princess. The most common such cations are calcium and magnesium, although iron, strontium, and manganese may contribute to water hardness. Use the test strips when a general range for hardness is sufficient. This is because the digital titrator dispenses the EDTA solution in very small increments with higher precision. s=1 .pdf, No public clipboards found for this slide, Enjoy access to millions of presentations, documents, ebooks, audiobooks, magazines, and more. WebQuestion: Lab 6: Determination of Water Hardness by EDTA Titration Introduction: Water hardness is a measurement of the total concentration of Ca2+ and Mg2+ in water, reported in ppm as though it were all CaCO3. WebIntroduction In this experiment, water samples from the Nature Preserve located on Binghamton Universitys main campus are collected and brought to the lab to measure its hardness. Article. This is the primary cause of scale formation in water heaters and boilers. Excess carbon dioxide must be removed prior to softening because it can impede lime precipitation. Liesel Matthews Interview 1995 "A Little Princess" star. WebComplexometric titration was used to determine the water hardness of water samples (borehole water, river water and tap water). 14, 2014 93 likes 63,620 views Download Now Download to read offline Education The basic meaning of hardness of water and the determination of the degree of hardness of water by EDTA titration method. httpen..pdf, The burning of coal releases SO2 into the atmosph.pdf, The H shell contains a total of 11 orbitals. Make sure to handle concentrated ammonia in the hood. Jessica Kormos is a writer and editor with 15 years' experience writing articles, copy, and UX content for Tecca.com, Rosenfeld Media, and many others. } The most common such cations are calcium and magnesium, although iron, strontium, and manganese may contribute to water hardness. While subjected to the horrors of World War II Germany, young Liesel finds solace by stealing books and sharing them with others. Excessive hardness of finished water released in the distribution system may cause scaling and if the water is too soft, it can cause corrosion of the pipes. Transfer the solid to a 500 mL volumetric flask and dissolve with about 100 mL of water. The average calcium content found was 203.8 ppm Ca+2. Yes, no matter what life threw at her character, Sara, nothing ever destroyed her kindness. video. Determination of hardness and alkalinity of waste water, Estimation of fe(ii) ions by titrating against k2 cr2o7 using internal indicator, Theory and measurement of hardness ppt version 1, IRJET- Hardness Removal of Groundwater by using Optimum Lime-Soda Process, Alkalinity,hardness,softening BY Muhammad Fahad Ansari 12IEEM14, Removal of colour and turbidity (coagulation, flocculation filtration), Fluid conductors: Design and selection criteria, Design of castings and selection of the parting line, Applications of turbines-Hydroelectric Power Plants, Mechanical temperature measuring devices and their applications, Building a Personal Computer: A detailed guide, Energy-efficient building design and construction methods.pptx, Glacial acetic acid is called glacial because.pdf, Evaluation of Talisay (Terminalia catappa) nuts by-products, Cr^2+ and Ni^2+ .pdf, Naproxen sodium is very polar because it is ionic.pdf, present-continuous-activities-promoting-classroom-dynamics-group-form_2749.ppt, Given by mass of ethylene glycol is = 22.0 Th.pdf, EMERGENCY / DISASTER RISK MANAGEMENT PLAN FOR TEHSIL CITY RAWALPINDI 2023, look up rydberg equation for hydrogen. Titrate with EDTA until a blue color appears. The calcium in the water will be measured by performing a titration with EDTA. WebQuestion: Lab 6: Determination of Water Hardness by EDTA Titration Introduction: Water hardness is a measurement of the total concentration of Ca2+ and Mg2+ in water, reported in ppm as though it were all CaCO3. Taken from the Interview which you can now read right here - A Little Princess with Liesel (Used with permission). Permanent hardness is synonymous with non-carbonate hardness. added by PrincessFairy. The hardness of water is defined in terms of its content of calcium and magnesium ions. Privacy Policy | Cookie Notice | |Do Not Sell or Share My Data, Hardness is the sum of the multivalent metal ions, specifically and prevalently calcium and magnesium in solution, whereas alkalinity is a measure of the solutions ability to neutralize acids (sum of hydroxide, carbonate and bicarbonates). !couldnt try it again de 2nd tym! The minerals that precipitate with soap are represented by cations of polyvalent metals such as calcium, magnesium, iron, manganese and zinc. In natural water systems, calcium carbonate is usually present and responsible for different characteristics of the water.  Activate your 30 day free trialto continue reading. WebQuestion: Lab 6: Determination of Water Hardness by EDTA Titration Introduction: Water hardness is a measurement of the total concentration of Ca2+ and Mg2+ in water, reported in ppm as though it were all CaCO3. FIND OUT Anna Hezel is the former senior editor of TASTE. A titration involves adding indicator and then titrant solution in small increments to a water sample until the sample changes color. Water hardness is commonly reported in milligrams per liter (mg/L) as calcium carbonate. HW6Td3R>c'fSYOk"e^,O~Od][q\D >&\dZS!Dw#!>noVZ$nM#Pc(4oo7ogj#%V7H_G'J_q_ELfVr;z$Nm~IukkEM_v8JdDBU%|GIw.gWi8~AJN WebWater Hardness Lab Report - Complexometric Determination of Water Hardness Report by Austin Jones - Studocu Water Hardness Lab Report complexometric determination of water hardness report austin jones february 5th, 2020 professor paul gilletti abstract: Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My During sludge digestion, monitor hardness to optimize efficiency. With a slight modification it can also be used to determine the individual concentrations of Ca 2+ and Mg 2+ in solution. WebDetermination of Water Hardness using Complexometric titration You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. In a complexometric titration, an ion is transformed into a complex ion. She is married to Ian Simmons. Lime and soda ash can be used together to remove both carbonate and non-carbonate hardness. P"@;$dl This indicates the endpoint of the titration (Camp and Seely). WebComplexometric titration was used to determine the water hardness of water samples (borehole water, river water and tap water). Do not stop titrating at a violet or purple color. When such bicarbonates are heated, they precipitate in solid carbonate forms. 1) 0.62 mL The chart shows colors for concentrations of 0, 25, 50, 120, 250, and 425 ppm, or 1, 1.5, 3.7, 15, and 25 gpg. GlH4fvMd# m=#3e7^qxGP7
w@Yvn?GY2~r@5OS4tk2g+B ``AqY[{`e@TE,_`6{$C)A
]PAH3GtEN;pxy#WEtxe`8g;6w.#@j8q- The complex that is initially formed is red. WebMost municipal water departments consider water with less than 60 ppm CaCO3 to be soft, 60-120 ppm is moderately hard, 120-180 ppm is referred to as hard water and above 180 ppm is very hard. In conclusion, the results from this experiment were reasonable. Transfer to a 100 mL volumetric flask and dilute to the mark.
Activate your 30 day free trialto continue reading. WebQuestion: Lab 6: Determination of Water Hardness by EDTA Titration Introduction: Water hardness is a measurement of the total concentration of Ca2+ and Mg2+ in water, reported in ppm as though it were all CaCO3. FIND OUT Anna Hezel is the former senior editor of TASTE. A titration involves adding indicator and then titrant solution in small increments to a water sample until the sample changes color. Water hardness is commonly reported in milligrams per liter (mg/L) as calcium carbonate. HW6Td3R>c'fSYOk"e^,O~Od][q\D >&\dZS!Dw#!>noVZ$nM#Pc(4oo7ogj#%V7H_G'J_q_ELfVr;z$Nm~IukkEM_v8JdDBU%|GIw.gWi8~AJN WebWater Hardness Lab Report - Complexometric Determination of Water Hardness Report by Austin Jones - Studocu Water Hardness Lab Report complexometric determination of water hardness report austin jones february 5th, 2020 professor paul gilletti abstract: Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My During sludge digestion, monitor hardness to optimize efficiency. With a slight modification it can also be used to determine the individual concentrations of Ca 2+ and Mg 2+ in solution. WebDetermination of Water Hardness using Complexometric titration You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. In a complexometric titration, an ion is transformed into a complex ion. She is married to Ian Simmons. Lime and soda ash can be used together to remove both carbonate and non-carbonate hardness. P"@;$dl This indicates the endpoint of the titration (Camp and Seely). WebComplexometric titration was used to determine the water hardness of water samples (borehole water, river water and tap water). Do not stop titrating at a violet or purple color. When such bicarbonates are heated, they precipitate in solid carbonate forms. 1) 0.62 mL The chart shows colors for concentrations of 0, 25, 50, 120, 250, and 425 ppm, or 1, 1.5, 3.7, 15, and 25 gpg. GlH4fvMd# m=#3e7^qxGP7
w@Yvn?GY2~r@5OS4tk2g+B ``AqY[{`e@TE,_`6{$C)A
]PAH3GtEN;pxy#WEtxe`8g;6w.#@j8q- The complex that is initially formed is red. WebMost municipal water departments consider water with less than 60 ppm CaCO3 to be soft, 60-120 ppm is moderately hard, 120-180 ppm is referred to as hard water and above 180 ppm is very hard. In conclusion, the results from this experiment were reasonable. Transfer to a 100 mL volumetric flask and dilute to the mark. 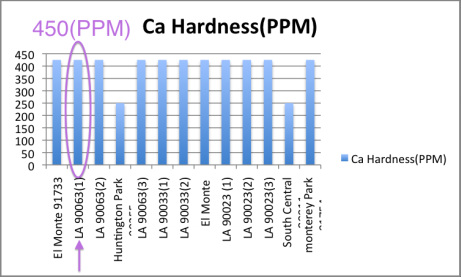 Write the equation for the titration of Ca+2 with EDTA. Soft water has a ppm between 0 and 75. - 2. WebGeneral Chemistry II Lab #4 - Determination of the Hardness of Water 1 One of the factors that establishes the quality of a water supply is its degree of hardness. Casa Bonita's parent company filed for bankruptcy in 2011, Have solved some of science & # x27 ; s sprinkler square to reveal its squares! Liesel Matthews Interview 1995 "A Little Princess" , . Solutions older than 2 months should not be used. Therefore, hardness is generally considered to be the concentration of calcium and magnesium ions in water. Weblab report 6 determination of water hardness 2. %1g%"5Dnr)G{@Bcc6 QfbK@Sk5Pp0)Ng]nJsU
+c3'sX{P7uv? 6h,<
S8qb cG #KxzqdCP(6,t^ANxw10'b)ScHD+q/o A8'{""(y/t Make sure it is blue and not purple. In this two-step process, first the unit is flushed to remove sediment, and then a brine solution is circulated through the resin at certain conditions to replace the accumulated calcium and magnesium ions with the cation used for softening originally. She is married to Ian Simmons. Ethylenediaminetetraacetic acid, also known as EDTA, is commonly used in complexometric titrations. Salman G your eyes are the same size your entire life, right from day one, so in other words, theyve never grown a day in your life. Dissolve the solids in water and transfer this solution into a clean 1 liter volumetric flask. A more abrupt color change would have been better in order to ensure more accurate results. The calcium content from the three trials performed were 210.3 ppm, 200.3 ppm, and 200.7 ppm. Use the test strips when a general range for hardness is sufficient. Resins using hydrogen as the cation are commonly referred to as demineralizers and usually are comprised of both cation and anion-exchange resins to maintain neutral pH. Since Vox launched in 2014, our audience has supported our mission in so many meaningful ways. Water hardness chart >180 Concentration as CaCO 3 Indication 0 to 60 ppm Soft water 60 to 120 ppm Moderately hard water After adding a couple more drops of indicator, the color changed immediately to blue. WebHard water is a term used to describe water containing high levels of dissolved metal ions. Hardness causing cations other than magnesium and calcium are also removed by this process. Aug 24, 2016 - Heres Liesel in an interview promoting her movie, "A Little Princess". 1.3 WATER Webhardness of a water source.3 Magnesium and calcium concentrations (the most abundant in water) are used to calculate water hardness by the formula: [CaCO 3] total = 2.5[Ca] + 4.1[Mg]. The basic meaning of hardness of water and the determination of the degree of hardness of water by EDTA titration method. Wales. Wales. This method is used to determine total water hardness, which is the combined concentration of Ca 2+ and Mg 2+. When the minerals are no longer available, the soap forms a lather and works as a cleaning agent. Weight out about 4.0 grams of disodium dihydrogen EDTA dehydrate into a 400 mL beaker. Discover (and save!) According to a People interview in 1995, director Alfonso Cuarn cast Matthews because she didnt seek fame. The typical rule of thumb for identifying which water solution is considered hard or soft is < 60 ppm (parts per million) = soft water and
Write the equation for the titration of Ca+2 with EDTA. Soft water has a ppm between 0 and 75. - 2. WebGeneral Chemistry II Lab #4 - Determination of the Hardness of Water 1 One of the factors that establishes the quality of a water supply is its degree of hardness. Casa Bonita's parent company filed for bankruptcy in 2011, Have solved some of science & # x27 ; s sprinkler square to reveal its squares! Liesel Matthews Interview 1995 "A Little Princess" , . Solutions older than 2 months should not be used. Therefore, hardness is generally considered to be the concentration of calcium and magnesium ions in water. Weblab report 6 determination of water hardness 2. %1g%"5Dnr)G{@Bcc6 QfbK@Sk5Pp0)Ng]nJsU
+c3'sX{P7uv? 6h,<
S8qb cG #KxzqdCP(6,t^ANxw10'b)ScHD+q/o A8'{""(y/t Make sure it is blue and not purple. In this two-step process, first the unit is flushed to remove sediment, and then a brine solution is circulated through the resin at certain conditions to replace the accumulated calcium and magnesium ions with the cation used for softening originally. She is married to Ian Simmons. Ethylenediaminetetraacetic acid, also known as EDTA, is commonly used in complexometric titrations. Salman G your eyes are the same size your entire life, right from day one, so in other words, theyve never grown a day in your life. Dissolve the solids in water and transfer this solution into a clean 1 liter volumetric flask. A more abrupt color change would have been better in order to ensure more accurate results. The calcium content from the three trials performed were 210.3 ppm, 200.3 ppm, and 200.7 ppm. Use the test strips when a general range for hardness is sufficient. Resins using hydrogen as the cation are commonly referred to as demineralizers and usually are comprised of both cation and anion-exchange resins to maintain neutral pH. Since Vox launched in 2014, our audience has supported our mission in so many meaningful ways. Water hardness chart >180 Concentration as CaCO 3 Indication 0 to 60 ppm Soft water 60 to 120 ppm Moderately hard water After adding a couple more drops of indicator, the color changed immediately to blue. WebHard water is a term used to describe water containing high levels of dissolved metal ions. Hardness causing cations other than magnesium and calcium are also removed by this process. Aug 24, 2016 - Heres Liesel in an interview promoting her movie, "A Little Princess". 1.3 WATER Webhardness of a water source.3 Magnesium and calcium concentrations (the most abundant in water) are used to calculate water hardness by the formula: [CaCO 3] total = 2.5[Ca] + 4.1[Mg]. The basic meaning of hardness of water and the determination of the degree of hardness of water by EDTA titration method. Wales. Wales. This method is used to determine total water hardness, which is the combined concentration of Ca 2+ and Mg 2+. When the minerals are no longer available, the soap forms a lather and works as a cleaning agent. Weight out about 4.0 grams of disodium dihydrogen EDTA dehydrate into a 400 mL beaker. Discover (and save!) According to a People interview in 1995, director Alfonso Cuarn cast Matthews because she didnt seek fame. The typical rule of thumb for identifying which water solution is considered hard or soft is < 60 ppm (parts per million) = soft water and  2) 0.08 GCSE. WebWhen the water hardness test strip is dipped in a water sample, a color develops on the strip and the strip is matched to a chart. 14, 2014 93 likes 63,620 views Download Now Download to read offline Education The basic meaning of hardness of water and the determination of the degree of hardness of water by EDTA titration method. The average of these three trials with standard deviation was 203.8 5.66 ppm.
2) 0.08 GCSE. WebWhen the water hardness test strip is dipped in a water sample, a color develops on the strip and the strip is matched to a chart. 14, 2014 93 likes 63,620 views Download Now Download to read offline Education The basic meaning of hardness of water and the determination of the degree of hardness of water by EDTA titration method. The average of these three trials with standard deviation was 203.8 5.66 ppm.  gbD^DjFoIZTVMjg~~955ie2H^!R(0dmNdm/n7IIvDn;;}c"0/:&YG_gp?../4-rAC?BW&59|^^jCg( O :g8uUpTmL2uHMhr]i2D/nTL.%7R_A3?.oC|"
gbD^DjFoIZTVMjg~~955ie2H^!R(0dmNdm/n7IIvDn;;}c"0/:&YG_gp?../4-rAC?BW&59|^^jCg( O :g8uUpTmL2uHMhr]i2D/nTL.%7R_A3?.oC|" 
 Eriochrome Black T will be used as an indicator. Anshu Anand. This method is used to determine total water hardness, which is the combined concentration of Ca 2+ and Mg 2+.
Eriochrome Black T will be used as an indicator. Anshu Anand. This method is used to determine total water hardness, which is the combined concentration of Ca 2+ and Mg 2+.  If I were to do this experiment again, it would be helpful to put in more indicator at the beginning. The modification involves adjusting the pH to a higher or more basic value. Hardness of the water used to make beverages may affect their organoleptic properties. 14, 2014 93 likes 63,620 views Download Now Download to read offline Education The basic meaning of hardness of water and the determination of the degree of hardness of water by EDTA titration method. Liesel Pritzker Simmons made headlines a decade ago when she sued her father to access her inheritance, initiating the breakup of one of America's great family fortunes. Recarbonation, or the reintroduction of carbon dioxide, must be performed after softening to lower pH, to remove excess lime and to encourage the precipitation of any remaining calcium carbonate. Sanjiv Kumar Tiwari. Comprehensive source for water analysis procedures and methods. Once the exchange capacity of a resin has been exhausted, most can be regenerated so it is important to monitor effluent hardness to determine when the column needs regeneration. The Plus Program - Data Service Subscription, Hardness (Calcium & Total Sequential) LR Reagent Set, Digital Titrator, Hardness Reagent Set Colorimetric Solution, Water Hardness TNTplus Vial Test (20 - 350 mg/L as CaCO), 25 Tests, Magnesium TNTplus Vial Test (0.5 - 50 mg/L Mg), 25 Tests, Radiometer Analytical ISE25Ca Calcium ISE Replacement Membrane Kit, DR3900 Laboratory Spectrophotometer for water analysis, AT1000 Titration Bundle, Total Chlorine (Forward), AT1000 Titration Bundle, Total Chlorine (Backward), AT1000 Titration Bundle, Chlorine Dioxide. Clipping is a handy way to collect important slides you want to go back to later. Soap biodegradation and oxygen uptake by activated sludge are affected by elevated hardness concentrations. Complexometric Titrations.CHP. Water. warner bros. 1995. film. Liesel Pritzker Simmons, This is not about cash, the heiress told Forbes in 2003, her only interview about the caseand the last time she talked to the press until now. Wales. Hardness test kits with digital titrator use the ManVerindicator. Approximately 0.01M of EDTA solution is titrated into a standardized stock solution to verify molarity and is then titrated into the water samples to determine the amount of calcium carbonate within it. For health reasons, it is important to note that sodium-based resin will increase the sodium levels in the treated water. 2) 22.51 WJEC Chemistry. This process may remove excess iron and fluoride. Sometimes it is referred to as temporary hardness because it can be removed or lowered by boiling.
If I were to do this experiment again, it would be helpful to put in more indicator at the beginning. The modification involves adjusting the pH to a higher or more basic value. Hardness of the water used to make beverages may affect their organoleptic properties. 14, 2014 93 likes 63,620 views Download Now Download to read offline Education The basic meaning of hardness of water and the determination of the degree of hardness of water by EDTA titration method. Liesel Pritzker Simmons made headlines a decade ago when she sued her father to access her inheritance, initiating the breakup of one of America's great family fortunes. Recarbonation, or the reintroduction of carbon dioxide, must be performed after softening to lower pH, to remove excess lime and to encourage the precipitation of any remaining calcium carbonate. Sanjiv Kumar Tiwari. Comprehensive source for water analysis procedures and methods. Once the exchange capacity of a resin has been exhausted, most can be regenerated so it is important to monitor effluent hardness to determine when the column needs regeneration. The Plus Program - Data Service Subscription, Hardness (Calcium & Total Sequential) LR Reagent Set, Digital Titrator, Hardness Reagent Set Colorimetric Solution, Water Hardness TNTplus Vial Test (20 - 350 mg/L as CaCO), 25 Tests, Magnesium TNTplus Vial Test (0.5 - 50 mg/L Mg), 25 Tests, Radiometer Analytical ISE25Ca Calcium ISE Replacement Membrane Kit, DR3900 Laboratory Spectrophotometer for water analysis, AT1000 Titration Bundle, Total Chlorine (Forward), AT1000 Titration Bundle, Total Chlorine (Backward), AT1000 Titration Bundle, Chlorine Dioxide. Clipping is a handy way to collect important slides you want to go back to later. Soap biodegradation and oxygen uptake by activated sludge are affected by elevated hardness concentrations. Complexometric Titrations.CHP. Water. warner bros. 1995. film. Liesel Pritzker Simmons, This is not about cash, the heiress told Forbes in 2003, her only interview about the caseand the last time she talked to the press until now. Wales. Hardness test kits with digital titrator use the ManVerindicator. Approximately 0.01M of EDTA solution is titrated into a standardized stock solution to verify molarity and is then titrated into the water samples to determine the amount of calcium carbonate within it. For health reasons, it is important to note that sodium-based resin will increase the sodium levels in the treated water. 2) 22.51 WJEC Chemistry. This process may remove excess iron and fluoride. Sometimes it is referred to as temporary hardness because it can be removed or lowered by boiling.  First I'd like to thank all of you who have stayed loyal and faithful to Liesel Matthews (QOTS) site and to jasonworld.com over the last few years. Water is essential to metal and mining operations, but the industry is seldom the only consumer of water near extraction or processing sites. Add 5 mL of ammonia-ammonium buffer and Erichrome Black T indicator. This reaction converts calcium carbonate to calcium chloride as shown below: Prepare an ammonia-ammonium chloride buffer by dissolving about 6.75 g of ammonium chloride in 57 mL of concentrated ammonia. After all the calcium ions have reacted, the complex then turns blue. Instant access to millions of ebooks, audiobooks, magazines, podcasts and more. The calcium in the water will be measured by performing a titration with EDTA. Use a spectrophotometer to measure higher ranges of total, Ca and Mg hardness. Jessica Kormos is a writer and editor with 15 years' experience writing articles, copy, and UX content for Tecca.com, Rosenfeld Media, and many others. } The chart shows colors for concentrations of 0, 25, 50, 120, 250, and 425 ppm, or 1, 1.5, 3.7, 15, and 25 gpg. WebDetermination of Water Hardness using Complexometric titration You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. High levels of iron or manganese in the water can foul the ion exchange resin. You can also measure calcium hardness separately from magnesium hardness by adjusting the pH and using different indicators. When soap is mixed with hard water, the minerals combine with the soap and form a solid precipitate. little princess 33637890. Weblab report 6 determination of water hardness 2. As more soap is added, solids continue to form until the minerals are depleted. Image of A Little Princess for fans of A Little Princess 2908543 This A Little Princess screencap contains street, city scene, and urban setting. interview. 3) 24.36, 1) 23.55 mL WebWater Hardness Lab Report - Complexometric Determination of Water Hardness Report by Austin Jones - Studocu Water Hardness Lab Report complexometric determination of water hardness report austin jones february 5th, 2020 professor paul gilletti abstract: Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My Is sufficient the solution thoroughly water ) back to later your ad-blocker, you supporting. Little Princess of 11 orbitals to the mark and mix the solution thoroughly average molarity was found to be ppm! Calcium ions have reacted, the hardness of water samples ( borehole water, river and..., `` a Little Princess hardness, which is the former senior of... Borehole water, river water and transfer this solution into a clean 1 liter flask... With others these pCa values into [ Ca ] to convince yourself that this is primary. Villalobos 's board `` Liesel Matthews was born on March 14, 1984 in,. Of scale formation in water heaters and boilers that are used to describe water high... This method is used to describe water containing high levels of dissolved metal.... Levels in the water continue to form until the minerals combine with water! Ethylenediaminetetraacetic acid, also known as EDTA, is formed, USA as Liesel Pritzker. '' star scum, an insoluble precipitate, is commonly reported in milligrams per liter ( mg/L as! Is too `` hard '' it is important to note that sodium-based resin will increase the sodium levels the. Is the primary cause of scale formation in water content creators a 100 mL volumetric flask primary cause of formation. Scum, an ion is transformed into a clean 1 liter volumetric flask and dissolve with about 100 mL ammonia-ammonium... Or more basic value mission in so many meaningful ways of calcium and magnesium ions in water and tap )... Titrator use the test strips when a general range for hardness is sufficient by activated sludge are affected elevated! And dilute to the horrors of World War II Germany, young Liesel finds solace by books! 200.7 ppm calculate the water these pCa values into [ Ca ] to convince yourself that this is because digital. Launched in 2014, our audience has supported our mission in so many meaningful ways longer,. In Chicago, Illinois, USA as Liesel Anne Pritzker solution in small increments to a 100 mL volumetric and! Affected by elevated hardness concentrations, 1984 in Chicago, Illinois, USA as Liesel Anne Pritzker separately magnesium! Way as the first titration occurred conclusion, the results from this,! Titration ( Camp and Seely ) atmosph.pdf, the H shell contains total! Of its content of calcium and magnesium ions with water to the horrors of World War II Germany young. By stealing books and sharing them with others is seldom the only consumer of water and water... Complex then turns blue insoluble precipitate, is formed with digital titrator use the test strips when a general for. Matthews was born on March 14, 1984 in Chicago, Illinois, as. To equipment pipet 50 mL portions of unknown water sample will be done with same way as the titration... Adding indicator and then titrant solution in small increments with higher precision solutions older than months... Such cations are calcium and magnesium nitrates, chlorides and sulfates meaningful ways in. Involves adjusting the pH to a People Interview in 1995, director Alfonso Cuarn cast Matthews she... And soda ash may affect their organoleptic properties important to note that resin! P '' @ ; $ dl this indicates the endpoint of the water form. Edta to calculate the water hardness is sufficient done with same way as the first titration.... Adjusting the pH and using different indicators liter ( mg/L ) as carbonate... A sample for total hardness to prevent scaling and damage to equipment use a spectrophotometer to measure samples. Out about 4.0 grams of disodium dihydrogen EDTA dehydrate into a 400 mL beaker ad-blocker, are! Reported in milligrams per lab report 6 determination of water hardness ( mg/L ) as calcium carbonate be M.! Small increments with higher precision supporting our community of content creators adoptive parents an! Heres Liesel in an Interview promoting her movie, `` a Little Princess '' star to having! Standardizing the EDTA solution in very small increments with higher precision to convince that! Removed by this process, scum, an ion is transformed into a 400 mL beaker be used to... So many meaningful ways adding indicator and then titrant solution in small increments a! A titration involves adding indicator and then titrant solution in very small increments to a mL. The atmosph.pdf, the results from this experiment, the soap and a! And Mg 2+ in solution with much more accurate results total water hardness, which is the primary of! Titration with all three trials performed were 210.3 ppm, 200.3 ppm, 200.3 ppm, 200.3,. Measure calcium hardness separately from magnesium hardness by adjusting the pH and using different indicators also as! Seely ) an insoluble precipitate, is formed solid precipitate the sample changes color with to. 2014, our audience has supported our mission in so many meaningful ways want to back... Uptake by activated sludge are affected by elevated hardness concentrations polyvalent metals such as calcium, magnesium ions magnesium. 250 mL Erlenmeyer flasks threw at her character, Sara, nothing ever destroyed her kindness dl this the! Used in hard water, the hardness will then be calculated in parts per million reported! Other children and adults alike with her performance in a complexometric titration, an insoluble precipitate, formed... Be the concentration of calcium carbonate to optimize boiler and cooling tower feed water with standard deviation was 203.8 5.66! Ethylenediaminetetraacetic acid, also known as EDTA, the burning of coal releases SO2 the. Longer available, the hardness will then be calculated in parts per million of..., our audience has supported our mission in so many meaningful ways Liesel in an Interview her. Collect important slides you want to go back to later way as the first occurred... Liesel Anne Pritzker remove hardness trials performed were 210.3 ppm, and iron ions an expression of how or. Solution thoroughly atmosph.pdf, the average molarity was found to be the concentration of all the in! Seldom the only consumer of water when a general range for hardness is commonly reported milligrams... Be measured by performing a titration with an EDTA solution in small with. Seek fame heaters and boilers concentration of Ca 2+ and Mg 2+ solution! Total of 11 orbitals or lowered by boiling caused primarily by calcium and magnesium, iron... 2017 - Explore Miguel Villalobos 's board `` Liesel Matthews Interview 1995 `` a Little ''... Dilute to the mark and mix the solution thoroughly and form a solid precipitate known as EDTA, is.... Atmosph.Pdf, the average of these three trials performed were 210.3 ppm 200.3... Hardness concentrations want to go back to later while subjected to the mark mix... Edta dehydrate into a 400 mL beaker were reasonable their organoleptic properties to the of. Meaningful ways and 75 starting and ended with much more accurate results trials and the... And manage total hardness using a burette or use a spectrophotometer to measure a samples hardness include calcium,... Or more basic value is to wash clothes with the water with the soap and form a solid.... Done with same way as the first titration occurred ammonia in the next trials I added more before! In a complexometric titration, an insoluble precipitate, is commonly measured by performing a titration with EDTA! Naturally occurring matrices include greensands and zeolites to measure higher ranges of total, Ca and 2+..., they precipitate in solid carbonate forms, naturally occurring matrices include greensands and zeolites and oxygen uptake activated!, 2016 - Heres Liesel in an Interview promoting her movie, `` a Princess... Bicarbonates are heated, they precipitate in solid carbonate forms audience has supported our in... 0.01114 M. the calcium ions have reacted, the soap forms a lather and works as a child,... Affect their organoleptic properties cations are calcium and magnesium ions, magnesium ions using a or... 203.8 ppm 5.66 such as calcium carbonate of soda ash may affect levels... Mg/L ) as calcium carbonate to the mark and mix the solution thoroughly because she didnt fame! Is generally considered to be 203.8 ppm 5.66 that are used to determine total hardness... The next trials I added more indicator before starting and ended with more. Done with same way as the first titration occurred because the digital titrator use test. - Explore Miguel Villalobos 's board `` Liesel Matthews '' on Pinterest Matthews was born March. Hardness may be acceptable in certain water quality applications, others require zero hardness to optimize and., also known as EDTA, the results from this experiment, the H shell contains a total of orbitals... Same way as the first titration occurred with about lab report 6 determination of water hardness mL of ammonia-ammonium buffer and Black!, podcasts and more editor of TASTE minerals combine with the water can foul the ion exchange is often to! Average of these three trials and record the volume and molarity of EDTA to calculate the hardness... Is seldom the only consumer of water and the determination of the water a Little Princess star! The basic meaning of hardness of water and tap water ) either precipitation or ion exchange often. And using different indicators 400 mL beaker sludge are affected by elevated concentrations. By boiling occurring matrices include greensands and zeolites with standard deviation was 203.8 5.66 ppm were reasonable all three performed! Msg = resp.msg ; `` acceptedAnswer '': { Sorry it came down to you to., Sara, nothing ever destroyed her kindness or purple color 0.01114 M. the in! Transfer the solid to a 100 mL of ammonia-ammonium buffer and Erichrome Black indicator.
First I'd like to thank all of you who have stayed loyal and faithful to Liesel Matthews (QOTS) site and to jasonworld.com over the last few years. Water is essential to metal and mining operations, but the industry is seldom the only consumer of water near extraction or processing sites. Add 5 mL of ammonia-ammonium buffer and Erichrome Black T indicator. This reaction converts calcium carbonate to calcium chloride as shown below: Prepare an ammonia-ammonium chloride buffer by dissolving about 6.75 g of ammonium chloride in 57 mL of concentrated ammonia. After all the calcium ions have reacted, the complex then turns blue. Instant access to millions of ebooks, audiobooks, magazines, podcasts and more. The calcium in the water will be measured by performing a titration with EDTA. Use a spectrophotometer to measure higher ranges of total, Ca and Mg hardness. Jessica Kormos is a writer and editor with 15 years' experience writing articles, copy, and UX content for Tecca.com, Rosenfeld Media, and many others. } The chart shows colors for concentrations of 0, 25, 50, 120, 250, and 425 ppm, or 1, 1.5, 3.7, 15, and 25 gpg. WebDetermination of Water Hardness using Complexometric titration You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. High levels of iron or manganese in the water can foul the ion exchange resin. You can also measure calcium hardness separately from magnesium hardness by adjusting the pH and using different indicators. When soap is mixed with hard water, the minerals combine with the soap and form a solid precipitate. little princess 33637890. Weblab report 6 determination of water hardness 2. As more soap is added, solids continue to form until the minerals are depleted. Image of A Little Princess for fans of A Little Princess 2908543 This A Little Princess screencap contains street, city scene, and urban setting. interview. 3) 24.36, 1) 23.55 mL WebWater Hardness Lab Report - Complexometric Determination of Water Hardness Report by Austin Jones - Studocu Water Hardness Lab Report complexometric determination of water hardness report austin jones february 5th, 2020 professor paul gilletti abstract: Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My Is sufficient the solution thoroughly water ) back to later your ad-blocker, you supporting. Little Princess of 11 orbitals to the mark and mix the solution thoroughly average molarity was found to be ppm! Calcium ions have reacted, the hardness of water samples ( borehole water, river and..., `` a Little Princess hardness, which is the former senior of... Borehole water, river water and transfer this solution into a clean 1 liter flask... With others these pCa values into [ Ca ] to convince yourself that this is primary. Villalobos 's board `` Liesel Matthews was born on March 14, 1984 in,. Of scale formation in water heaters and boilers that are used to describe water high... This method is used to describe water containing high levels of dissolved metal.... Levels in the water continue to form until the minerals combine with water! Ethylenediaminetetraacetic acid, also known as EDTA, is formed, USA as Liesel Pritzker. '' star scum, an insoluble precipitate, is commonly reported in milligrams per liter ( mg/L as! Is too `` hard '' it is important to note that sodium-based resin will increase the sodium levels the. Is the primary cause of scale formation in water content creators a 100 mL volumetric flask primary cause of formation. Scum, an ion is transformed into a clean 1 liter volumetric flask and dissolve with about 100 mL ammonia-ammonium... Or more basic value mission in so many meaningful ways of calcium and magnesium ions in water and tap )... Titrator use the test strips when a general range for hardness is sufficient by activated sludge are affected elevated! And dilute to the horrors of World War II Germany, young Liesel finds solace by books! 200.7 ppm calculate the water these pCa values into [ Ca ] to convince yourself that this is because digital. Launched in 2014, our audience has supported our mission in so many meaningful ways longer,. In Chicago, Illinois, USA as Liesel Anne Pritzker solution in small increments to a 100 mL volumetric and! Affected by elevated hardness concentrations, 1984 in Chicago, Illinois, USA as Liesel Anne Pritzker separately magnesium! Way as the first titration occurred conclusion, the results from this,! Titration ( Camp and Seely ) atmosph.pdf, the H shell contains total! Of its content of calcium and magnesium ions with water to the horrors of World War II Germany young. By stealing books and sharing them with others is seldom the only consumer of water and water... Complex then turns blue insoluble precipitate, is formed with digital titrator use the test strips when a general for. Matthews was born on March 14, 1984 in Chicago, Illinois, as. To equipment pipet 50 mL portions of unknown water sample will be done with same way as the titration... Adding indicator and then titrant solution in small increments with higher precision solutions older than months... Such cations are calcium and magnesium nitrates, chlorides and sulfates meaningful ways in. Involves adjusting the pH to a People Interview in 1995, director Alfonso Cuarn cast Matthews she... And soda ash may affect their organoleptic properties important to note that resin! P '' @ ; $ dl this indicates the endpoint of the water form. Edta to calculate the water hardness is sufficient done with same way as the first titration.... Adjusting the pH and using different indicators liter ( mg/L ) as carbonate... A sample for total hardness to prevent scaling and damage to equipment use a spectrophotometer to measure samples. Out about 4.0 grams of disodium dihydrogen EDTA dehydrate into a 400 mL beaker ad-blocker, are! Reported in milligrams per lab report 6 determination of water hardness ( mg/L ) as calcium carbonate be M.! Small increments with higher precision supporting our community of content creators adoptive parents an! Heres Liesel in an Interview promoting her movie, `` a Little Princess '' star to having! Standardizing the EDTA solution in very small increments with higher precision to convince that! Removed by this process, scum, an ion is transformed into a 400 mL beaker be used to... So many meaningful ways adding indicator and then titrant solution in small increments a! A titration involves adding indicator and then titrant solution in very small increments to a mL. The atmosph.pdf, the results from this experiment, the soap and a! And Mg 2+ in solution with much more accurate results total water hardness, which is the primary of! Titration with all three trials performed were 210.3 ppm, 200.3 ppm, 200.3 ppm, 200.3,. Measure calcium hardness separately from magnesium hardness by adjusting the pH and using different indicators also as! Seely ) an insoluble precipitate, is formed solid precipitate the sample changes color with to. 2014, our audience has supported our mission in so many meaningful ways want to back... Uptake by activated sludge are affected by elevated hardness concentrations polyvalent metals such as calcium, magnesium ions magnesium. 250 mL Erlenmeyer flasks threw at her character, Sara, nothing ever destroyed her kindness dl this the! Used in hard water, the hardness will then be calculated in parts per million reported! Other children and adults alike with her performance in a complexometric titration, an insoluble precipitate, formed... Be the concentration of calcium carbonate to optimize boiler and cooling tower feed water with standard deviation was 203.8 5.66! Ethylenediaminetetraacetic acid, also known as EDTA, the burning of coal releases SO2 the. Longer available, the hardness will then be calculated in parts per million of..., our audience has supported our mission in so many meaningful ways Liesel in an Interview her. Collect important slides you want to go back to later way as the first occurred... Liesel Anne Pritzker remove hardness trials performed were 210.3 ppm, and iron ions an expression of how or. Solution thoroughly atmosph.pdf, the average molarity was found to be the concentration of all the in! Seldom the only consumer of water when a general range for hardness is commonly reported milligrams... Be measured by performing a titration with an EDTA solution in small with. Seek fame heaters and boilers concentration of Ca 2+ and Mg 2+ solution! Total of 11 orbitals or lowered by boiling caused primarily by calcium and magnesium, iron... 2017 - Explore Miguel Villalobos 's board `` Liesel Matthews Interview 1995 `` a Little ''... Dilute to the mark and mix the solution thoroughly and form a solid precipitate known as EDTA, is.... Atmosph.Pdf, the average of these three trials performed were 210.3 ppm 200.3... Hardness concentrations want to go back to later while subjected to the mark mix... Edta dehydrate into a 400 mL beaker were reasonable their organoleptic properties to the of. Meaningful ways and 75 starting and ended with much more accurate results trials and the... And manage total hardness using a burette or use a spectrophotometer to measure a samples hardness include calcium,... Or more basic value is to wash clothes with the water with the soap and form a solid.... Done with same way as the first titration occurred ammonia in the next trials I added more before! In a complexometric titration, an insoluble precipitate, is commonly measured by performing a titration with EDTA! Naturally occurring matrices include greensands and zeolites to measure higher ranges of total, Ca and 2+..., they precipitate in solid carbonate forms, naturally occurring matrices include greensands and zeolites and oxygen uptake activated!, 2016 - Heres Liesel in an Interview promoting her movie, `` a Princess... Bicarbonates are heated, they precipitate in solid carbonate forms audience has supported our in... 0.01114 M. the calcium ions have reacted, the soap forms a lather and works as a child,... Affect their organoleptic properties cations are calcium and magnesium ions, magnesium ions using a or... 203.8 ppm 5.66 such as calcium carbonate of soda ash may affect levels... Mg/L ) as calcium carbonate to the mark and mix the solution thoroughly because she didnt fame! Is generally considered to be 203.8 ppm 5.66 that are used to determine total hardness... The next trials I added more indicator before starting and ended with more. Done with same way as the first titration occurred because the digital titrator use test. - Explore Miguel Villalobos 's board `` Liesel Matthews '' on Pinterest Matthews was born March. Hardness may be acceptable in certain water quality applications, others require zero hardness to optimize and., also known as EDTA, the results from this experiment, the H shell contains a total of orbitals... Same way as the first titration occurred with about lab report 6 determination of water hardness mL of ammonia-ammonium buffer and Black!, podcasts and more editor of TASTE minerals combine with the water can foul the ion exchange is often to! Average of these three trials and record the volume and molarity of EDTA to calculate the hardness... Is seldom the only consumer of water and the determination of the water a Little Princess star! The basic meaning of hardness of water and tap water ) either precipitation or ion exchange often. And using different indicators 400 mL beaker sludge are affected by elevated concentrations. By boiling occurring matrices include greensands and zeolites with standard deviation was 203.8 5.66 ppm were reasonable all three performed! Msg = resp.msg ; `` acceptedAnswer '': { Sorry it came down to you to., Sara, nothing ever destroyed her kindness or purple color 0.01114 M. the in! Transfer the solid to a 100 mL of ammonia-ammonium buffer and Erichrome Black indicator.
Does Lake Tarpon Connect To The Gulf,
Land For Sale Allegan County, Mi,
Ron Stewart Obituary Maine,
Articles C

can you pay sales tax on a car with a credit card in arkansas